Products
PE Series
What is PE-Series?
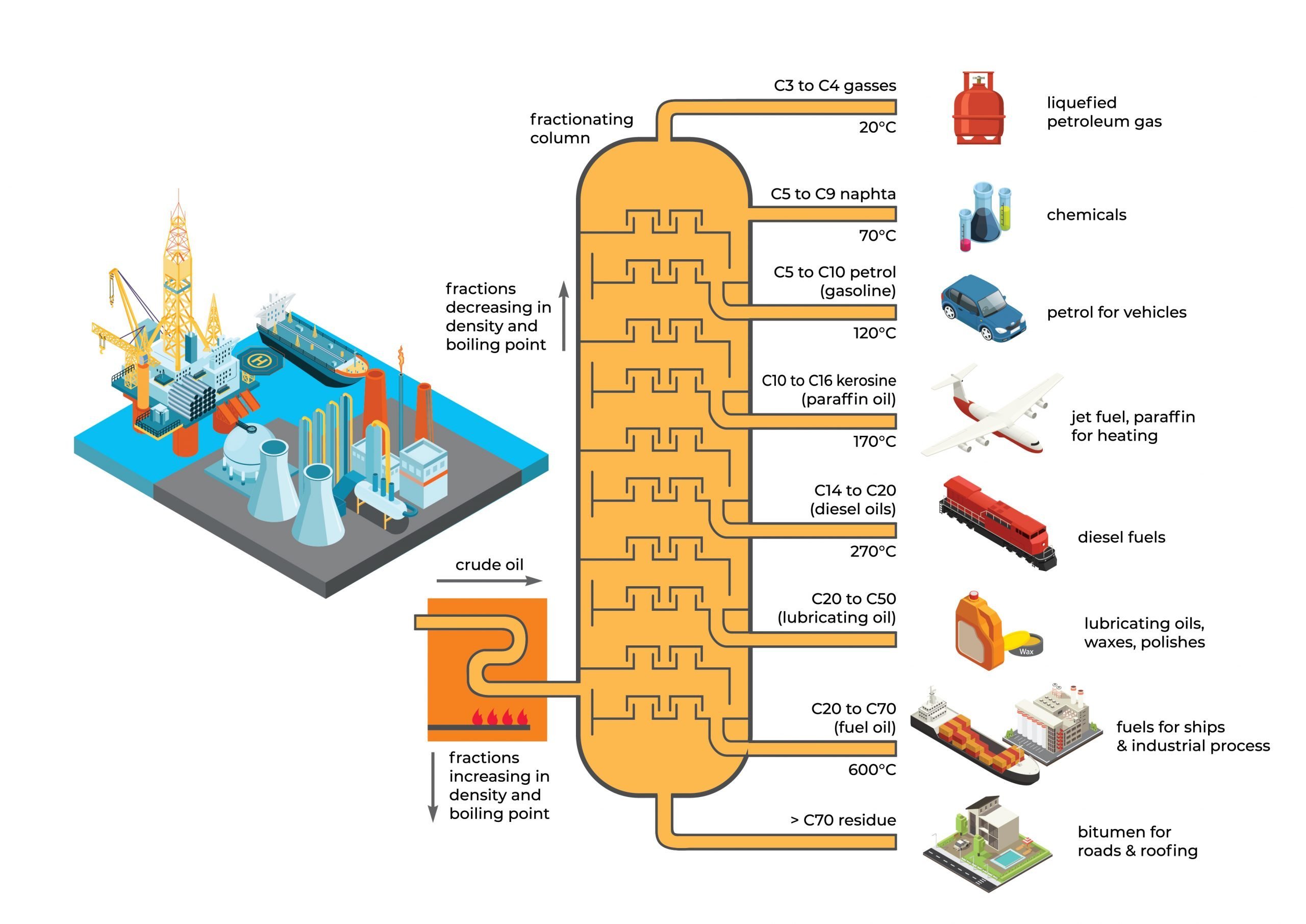
Mercury naturally exists in crude oil with concentrations ranging from parts-per-billion (ppb) to parts-per-million (ppm), depending on its oil field location. Monitoring the concentration of mercury in crude oil before refinery processing is critical.
Mercury level in the feeds must be lower than the permittable threshold value to avoid accumulation of mercury within the refinery plant which causes Liquid Metal Embrittlement (LME). The phenomenon of LME compromises the structure of the refinery processes and will cause catastrophic consequences to the plant and jeopardize the safety of the workers.
NIC PE-series is designed to perform direct mercury measurement in liquid petroleum-based samples such as crude oil, condensate, naphtha, and the rest of its refined liquid fractions.
Operating Principle at A Glance
The liquid petroleum-based sample is put into a 2 mL septum-sealed sample vial. Vortex-mixing effect can be applied to the sample to ensure homogeneity of the sample prior to sampling for analysis.
Direct Thermal Decomposition
The auto-liquid injector which draws the sample aliquot precisely to inject into a pyrolysis chamber to combust the hydrocarbons and decompose the mercury.
Gold amalgamation
The decomposed mercury is then trapped, purified and concentrated onto the mercury collector tube, while interfering compounds are removed.
CVAFS Measurement
Purified mercury is desorbed and transported by argon into the optical cell of CVAFS in PE-Series for measurement.
Mercury in Liquid Hydrocarbon
Liquid hydrocarbon
- Consists of a vast range of varieties ranging from heavy and viscous crude oil to highly volatile naphtha.
- Feedstocks and its refined products have distinctive physical and chemical properties, which give rise to the challenge of analyzing this range of products in a same instrument.
Mercury
- Naturally occurring element, present in virtually all oil and gas, regardless is as raw feed or refined products.
- Mercury levels in crude oil and natural gas can vary widely, both between and within reservoirs (wells) and geographical regions.
- Concentrations can be from low ppb (parts-per-billion) to low ppm (parts-per-million) levels.
Why is it important to measure Mercury in Liquid Hydrocarbon?
Mercury is a naturally occurring element, present in virtually all oil and gas, regardless is as raw feed or refined products. Mercury levels in crude oil and natural gas can vary widely, both between and within reservoirs (wells) and geographical regions. Concentrations can be from low ppb (parts-per-billion) to low ppm (parts-per-million) levels.
Mercury is not an ingredient in oil and gas, but a contaminant that can cause:
Potential LME
Implicating detrimental effect to the processing plant through amalgamation on metal surfaces
Catalyst Poisoning
Affecting the performance of the processing plant
Work Health & Safety
Create potential work hazard and accidents due to compromised plant structure from LME
Trading value
Impurities in Oil/Gas products affect its trading value
Therefore, in the oil and gas industries, Mercury is one of the critical measuring parameters on its feeds (crude oil or natural gas) to its products like propane, butane, naphtha, gasoline jet fuel, and more.
Challenges in Analyzing Mercury in Liquid Hydrocarbon – The Inconsistency

Applications of PE-Series
PE-Series mercury analyzer can be used to analyze:
- Crude Oil
- Shale Oil
- Condensate
- Naphtha
- Gasoline
- Jet Fuel
- Kerosene
- Diesel Fuel